COVID-19 testing by SR Scientific
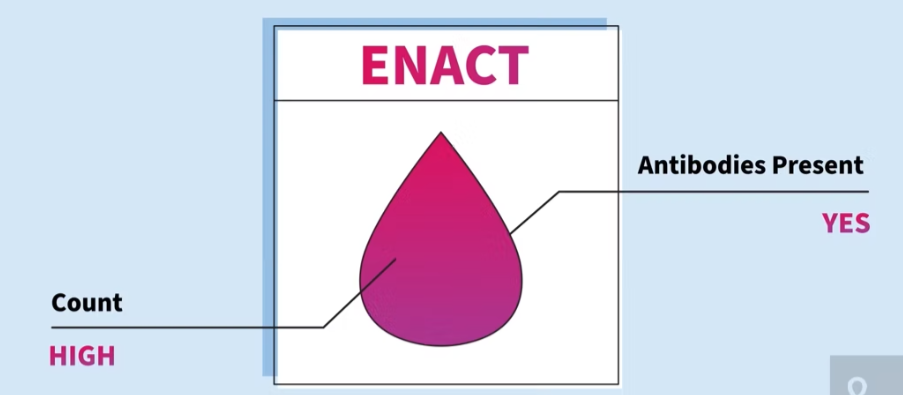
SR Scientific offers COVID-19 PCR, COVID-19 antibody, and respiratory pathogens panel tests with accurate results, typically within 24 hours. SR Scientific can serve patients with or without insurance. SR Scientific prides itself on its experienced, responsive, and compassionate lab personnel and leadership who are dedicated to superior customer service.
Facilities and organizations
Since the initial outbreak, SR Scientific has focused on providing care to the most vulnerable populations: retirement communities, skilled nursing facilities, and personal care communities.
SR Scientific also helps employers groups, governments and education institutions stay healthy with programs to respond on-site and on-demand to keep their populations healthy.
SR Scientific is helping patients take the precautionary steps needed to reduce the risk of exposure by servicing the most vulnerable population. Testing can help reduce the spread of COVID-19 and reduce the overall number of hospitalizations. This results in better health for patients and significant savings.